
Lithium metal is soft enough to be cut with a knife. Molten lithium is significantly more reactive than its solid form. Lithium's low reactivity is due to the proximity of its valence electron to its nucleus (the remaining two electrons are in the 1s orbital, much lower in energy, and do not participate in chemical bonds). Because of this, lithium is a good conductor of heat and electricity as well as a highly reactive element, though it is the least reactive of the alkali metals. Like the other alkali metals (which are sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr)), lithium has a single valence electron that, in the presence of solvents, is easily released to form Li +. The alkali metals are also called the lithium family, after its leading element. Properties Atomic and physical Lithium ingots with a thin layer of black nitride tarnish Lithium-based drugs are useful as a mood stabilizer and antidepressant in the treatment of mental illness such as bipolar disorder.
It has no established metabolic function. Lithium is present in biological systems in trace amounts. These uses consume more than three-quarters of lithium production. Lithium and its compounds have several industrial applications, including heat-resistant glass and ceramics, lithium grease lubricants, flux additives for iron, steel and aluminium production, lithium metal batteries, and lithium-ion batteries. The transmutation of lithium atoms to helium in 1932 was the first fully human-made nuclear reaction, and lithium deuteride serves as a fusion fuel in staged thermonuclear weapons. For related reasons, lithium has important uses in nuclear physics. Because of its relative nuclear instability, lithium is less common in the solar system than 25 of the first 32 chemical elements even though its nuclei are very light: it is an exception to the trend that heavier nuclei are less common. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes found in nature have among the lowest binding energies per nucleon of all stable nuclides. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride.

Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. It does not occur freely in nature, but occurs mainly as pegmatitic minerals, which were once the main source of lithium. It corrodes quickly in air to a dull silvery gray, then black tarnish. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. Under standard conditions, it is the least dense metal and the least dense solid element. It is a soft, silvery-white alkali metal.

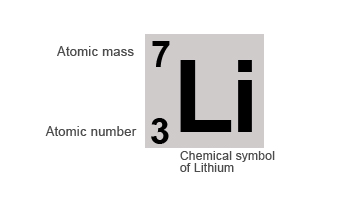
Lithium (from Ancient Greek λίθος ( líthos) 'stone') is a chemical element with the symbol Li and atomic number 3.


 0 kommentar(er)
0 kommentar(er)
